- Suggested reading
- Reading guide
- Need to Know
There are two chapters for suggested readings, from Lippincott, these two chapters are pretty succinct for the topics at hand. Chapter 13 is on the pentose phosphate pathway, and Chapter 14 is on glycosaminoglycans, proteoglycans, and glycoproteins (this may seem a bit out of place, but it’s not!). The short descriptions listed below are some of the very big-picture, high-yield points. The reading and the class session will go into more depth and detail. The reading guides are also posted.
As I have said in other modules, this reading guide is for people who have a difficult time focusing on these types of chapters. Most students DO NOT need to use the guide.
Diagram and describe the pentose phosphate pathway and its role in other metabolic pathways
-
Purpose of the pentose phosphate pathway
-
Where is NADPH/H used?
-
Glucose 6-phosphate dehydrogenase (G6PDH)
-
Transketolase
Describe a cause of hemolytic anemia as it relates to the pentose phosphate pathway
-
Glucose 6-phosphate dehydrogenase (G6PDH)
-
Glucose 6-phosphate dehydrogenase deficiency
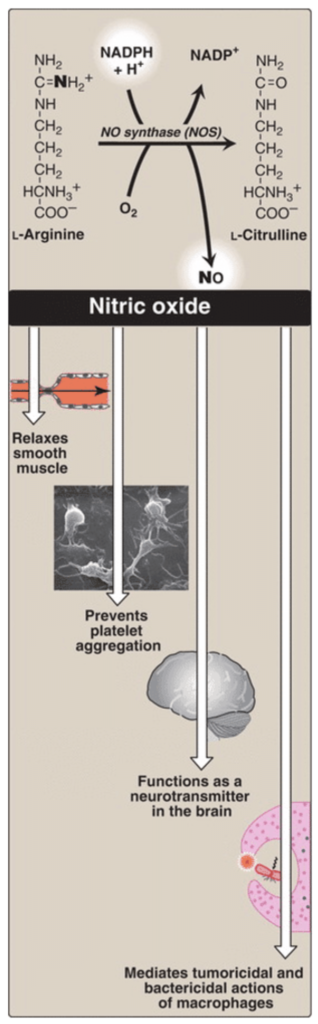
Describe how the pentose phosphate pathway is important in the synthesis of nitric oxide.
-
(This is straightforward.)
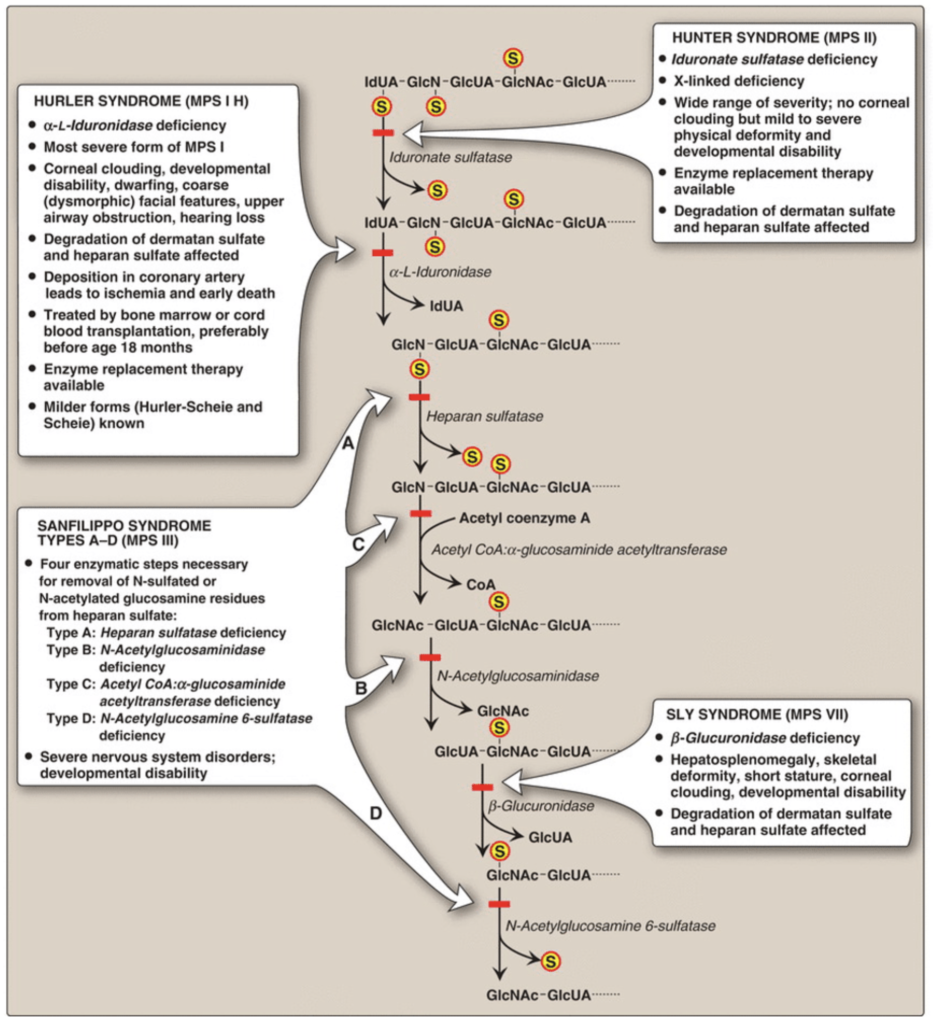
Diagram the basic features of glycosaminoglycans (GAGs) and proteoglycans and describe the basis of diseases that result from defects in the degradation pathway of GAGs
-
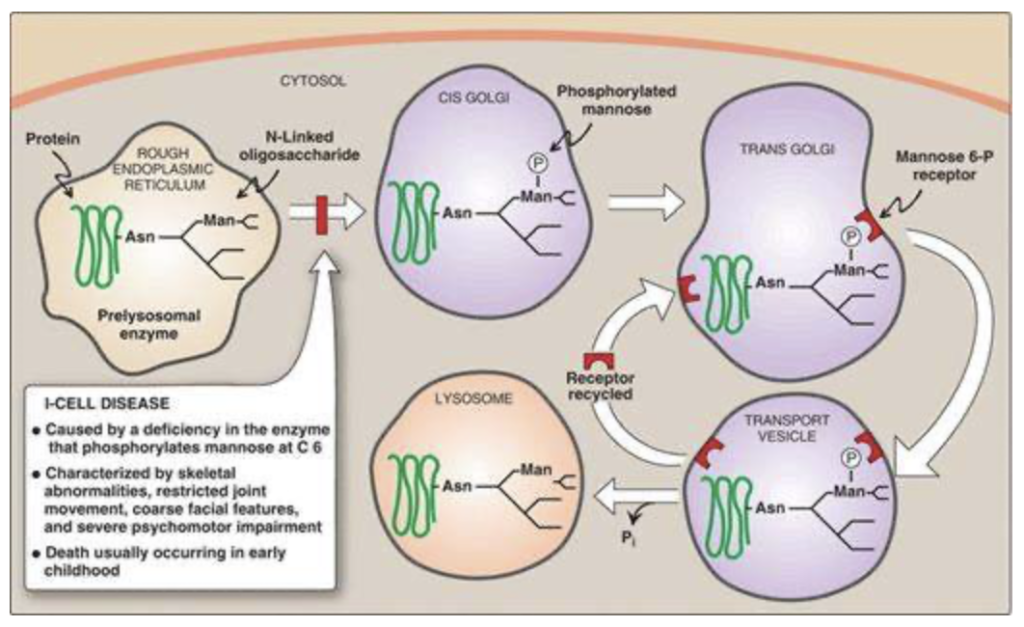
I-cell disease
-
Mucopolysaccharidoses
Diagram and describe the pentose phosphate pathway and its role in other metabolic pathways
Osmosis video on the pentose phosphate pathway.
Describe a cause of hemolytic anemia as it relates to the pentose phosphate pathway
This is a very high-yield thing to remember. Therefore, Osmosis has a video on it.
Describe how the pentose phosphate pathway is important in the synthesis of nitric oxide
Diagram the basic features of glycosaminoglycans (GAGs) and proteoglycans and describe the basis of diseases that result from defects in the degradation pathway of GAGs
The heteropolysaccharide chains are elongated by the sequential addition of alternating acidic and amino sugars donated primarily by their uridine diphosphate (UDP) derivatives. The reactions are catalyzed by a family of specific glycosyltransferases. Because GAGs are produced for export from the cell, their synthesis occurs primarily in the Golgi and not in the cytosol. (Taken from Lippincott Illustrated Reviews: Biochemistry, 7e.)
GAG is degraded in lysosomes, which contain hydrolytic enzymes that are most active at a pH of ~5. Therefore, as a group, these enzymes are called acid hydrolases. [Note: The low pH optimum is a protective mechanism that prevents the enzymes from destroying the cell should leakage occur into the cytosol where the pH is neutral.] The half-lives of GAG vary from minutes to months and are influenced by the type of GAG and its location in the body. (Taken from Lippincott Illustrated Reviews: Biochemistry, 7e.)
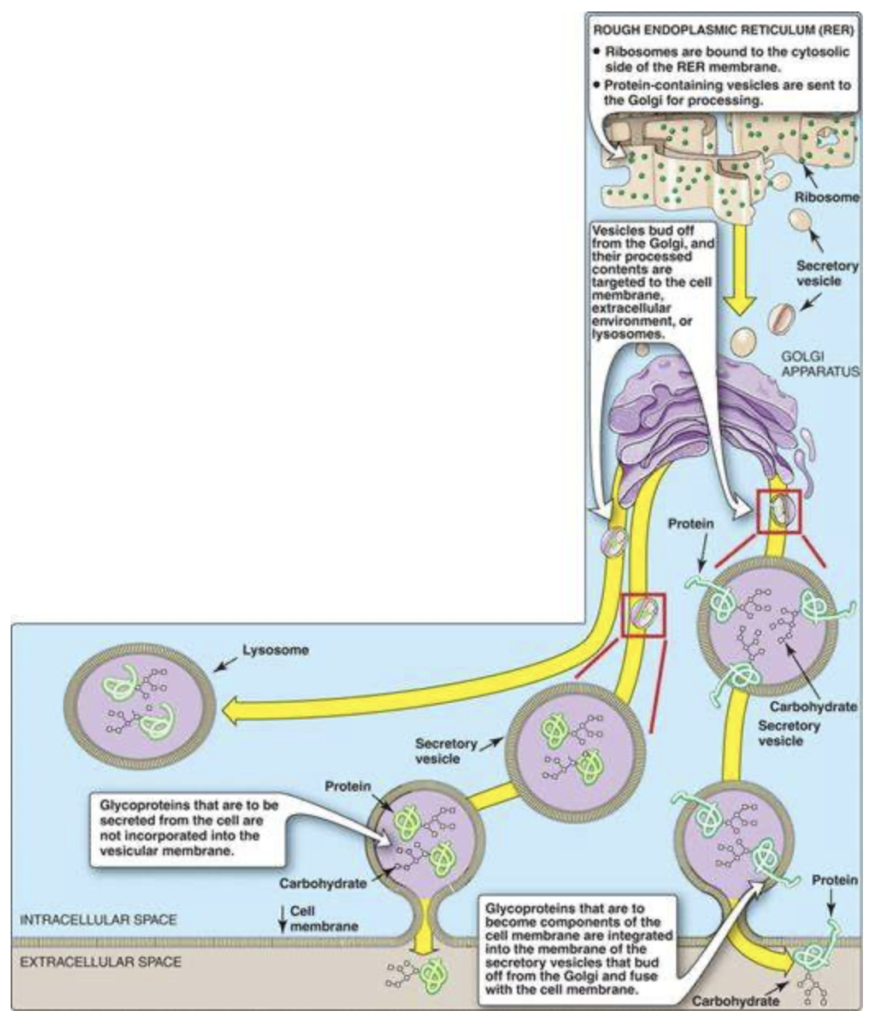
Describe the synthesis of glycoproteins and the importance of modifying protein with sugar
Glycoproteins, that are destined for cellular membranes, lysosomes, or to be exported from the cell, are synthesized on ribosomes attached to the RER. These proteins contain specific signal sequences that act as molecular addresses, targeting the proteins to their proper destinations.
A high-yield disease to know that is related to glycoproteins is I-cell disease. This disease results from a defect in the addition of the mannose 6-phosphate tag on enzymes destined for the lysosome (see the figure below). As such, these hydrolytic enzymes end up being secreted from the cell. Substrates within lysosomes accumulate, resulting in large inclusion bodies, hence I-cells. Patients have skeletal abnormalities, joint impairment, coarse facial features, and psychomotor impairment culminating in death by the age of 8 years.